Abstract
Background Renal impairment (RI) is frequently presented in multiple myeloma (MM) patients, indicating inferior survival and high early death risk. Renal response is associated with hematological response and overall survival in MM patients. Bortezomib-based triplet regimens have been recommended as first-line therapy for MM with RI. Pomalidomide has also been proved with significant efficacy in these special patients. However, the triplet combination of pomalidomide with bortezomib and dexamethasone (PVD) has not been prospectively evaluated in MM patients with RI. This prospective, open-label, multicenter, phase 2 study (ChiCTR2100043748) was designed to evaluate the renal response of PVD regimen as first-line therapy in newly diagnosed MM (NDMM) with RI.
Methods NDMM patients with MM-related RI (defined as estimated glomerular filtration rate (eGFR) < 40ml/min) who had measurable disease were enrolled in the study. RI caused by other reasons than tubular nephropathy was excluded. Patients received a maximum of 9 cycles of PVD therapy. Autologous stem cell transplantation (ASCT) was administrated after 3 to 6 PVD cycles for transplant-eligible patients. Primary endpoint was renal ORR at 3 months. Secondary endpoints included best renal response, hematological response, minimal residual disease (MRD), progression-free survival (PFS), overall survival (OS) and safety. Both hematological response and renal response were assessed by International Myeloma Working Group (IMWG) criteria.
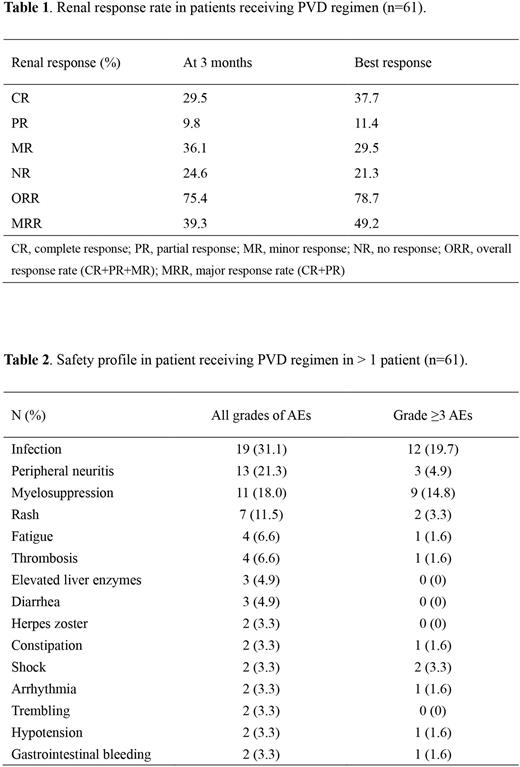
Results Between Feb 28, 2021 and Jan 21, 2022, sixty-one patients were enrolled across 28 hospitals, with a median age of 61 years (range: 38-79). The proportion of R-ISS stage III was 56.9%. Median serum creatine level and eGFR were 349 μmol/L (range: 155-1718) and 12.9 mL/min (range: 2.5-36.3), respectively. Twelve (19.7%) patients received dialysis. By the data cut-off date of July 15, 2022, median number of PVD treatment cycles was 5 (range: 1-9). Twelve patients had received ASCT. The primary endpoint with renal ORR at 3 months was 75.4% (39.3% ≥ renal-PR, 29.5% renal-CR). Renal responses deepened over time, with the best renal ORR was 78.7% (49.1% ≥ renal-PR, 37.7% renal-CR) (Table 1). Dialysis independence rate was 66.7% (8/12). The ORR of best hematological response was 91.8% (77.0% ≥VGPR, 55.7% CR). Within 20 patients who have finished 9 cycles of PVD therapy or ASCT, MRD detection by next-generation flow cytometry (NGF) was performed in 15 patients, while 13 (86.7%) had reached MRD negative. The most common adverse effects (incidence >10%) were infection (31.1%), peripheral neuritis (21.3%), myelosuppression (18.0%) and skin rash (11.5%) (Table 2). Median follow-up time was 11 months. Median PFS and OS have still not been reached.
Conclusion This study demonstrated favorable safety and efficacy results of PVD regimen for NDMM patients that are consistent with the initial report, with a high renal response and a significant MRD negative rate.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal